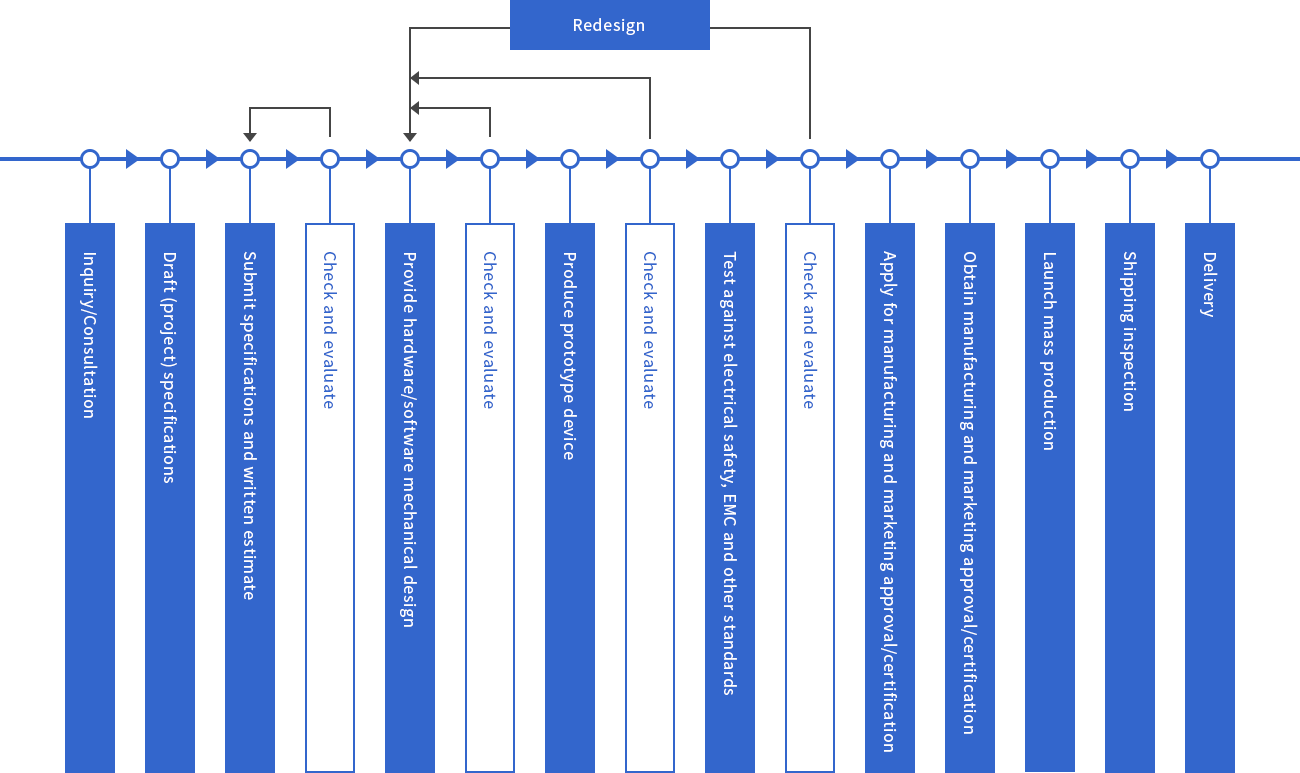
Technologies developed and manufactured by CME
From our inception, CME has developed and manufactured an array of quality medical electronics. We are especially proficient in developing the control components at the core of electronic devices, while our circuit and firmware design has won accolades across a wide customer base. CME also develops and manufactures quality non-electronic medical devices.
Safety and effectiveness are our guiding watchwords. Creating outstanding products that meet customer requirements at every stage is our mission. Thus, we accommodate reworked specifications and other changes during the development process with speed and agility, working toward our ultimate product vision: true customer satisfaction.
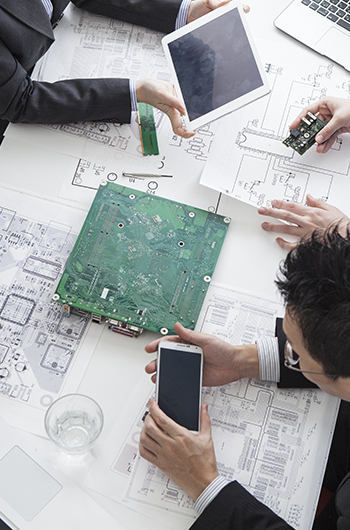
Electronic medical equipment must clear many more regulatory hurdles than standard electronics. Specifically, manufacturers are required to certify that the equipment complies with product class standards in areas including electrical safety and electromagnetic compatibility (EMC).
At CME, we boast superior expertise with standards such as JIS T0601-1-2, required for the certification and approval of most electronic medical devices. From the initial design stage and throughout development, we ensure full compliance with all standards.。
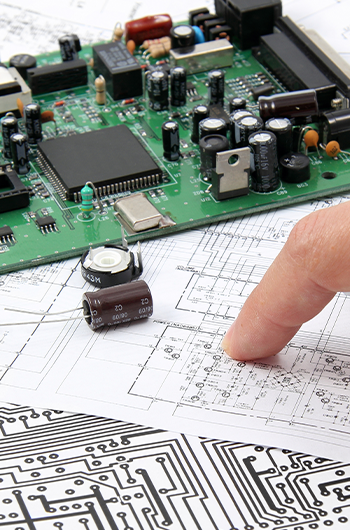
As a full-spectrum medical equipment manufacturer, CME is one of a limited number of qualified facilities that the government permits to produce and ship finished medical devices directly to vendors. Our comprehensive production and quality management system has been developed in full compliance with Japan’s Pharmaceutical Affairs Law and international standards published by the ISO.
CME’s medical equipment manufacturing license also permits us to manufacture surgical equipment, classified as highly controlled (Class III) medical devices.
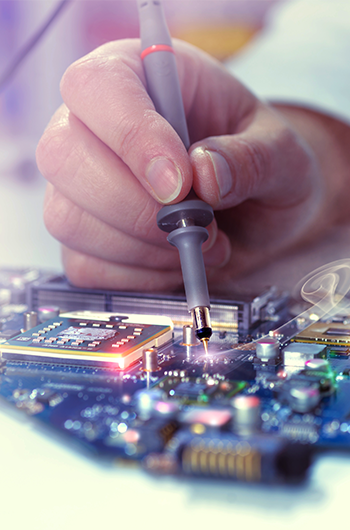
Nothing is more important to us than product quality. To assure that everything we make is the best it can be, we have implemented a quality management system in accordance with ISO 13485:2016, the international standard for medical device manufacturing. Our system conforms with Ministry of Health, Labour and Welfare QMS ordinances, as specified in the Japan Pharmaceutical Affairs Law.
The CME quality management system governs every step in the manufacturing process, from materials procurement onward. In obtaining ISO13485 certification from an independent, internationally-recognized standards body, we have demonstrated that we meet stringent international quality standards in all phases of our operation.

Companies seeking to sell medical equipment in the Japanese market must first obtain government marketing approval or certification. As the holder of a first-class license as a Marketing Authorization Holder (MAH), CME is qualified to apply for marketing and manufacturing authorization for the gamut of medical devices, including those classified as specially controlled (Class IV) in the Japanese regulatory scheme.
In some cases, our customers hold a medical device marketing permit without the manufacturing authorization, and may not be qualified to submit for regulatory approval themselves. In this scenario, CME can file for and obtain the authorization. Then, in our OEM manufacturing capacity, we produce and supply the products to customer specifications. Our integrated service and support, from document preparation through discussion with the regulatory authorities, makes the approval process faster and smoother.

CME is the catalyst for effective medical-engineering collaboration. Medical and engineering professionals at universities and other educational research institutions, work synergistically with private-sector counterparts to research and develop new technologies and create new businesses in the medical field, with CME mediating the interplay and collaboration process.
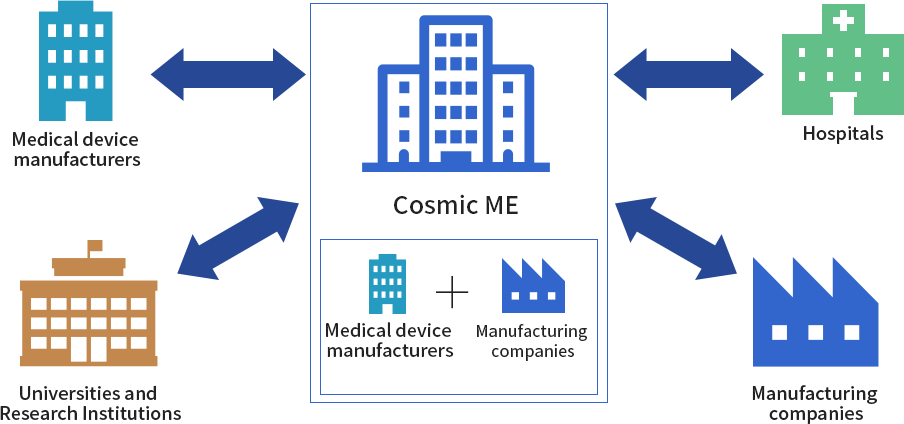
Medical schools and institutions, among many others who depend on medical devices, constantly seek new treatment modalities or improvements on existing therapies. Meanwhile, on the engineering side, each science and engineering department and manufacturing firm has unique technology and know-how which could make that happen. In short, the medical field has the needs, and the engineering field has the seeds.
Unfortunately, Japan hasn’t developed the ecosystem for the symbiotic relationship between the two to take shape naturally.
International players face particularly daunting challenges in the Japanese system. Government policy focuses on supporting domestic medical device production, and cultivating the export market for these domestic players. However, in the opposite direction, international firms entering the Japanese medical field must deal with numerous obstacles, starting with the difficulty they face in trying to obtain business licenses. We serve to support companies who might otherwise hesitate to enter Japan’s medical market, by confidently guiding them past potential regulatory hurdles and other bumps in the road.
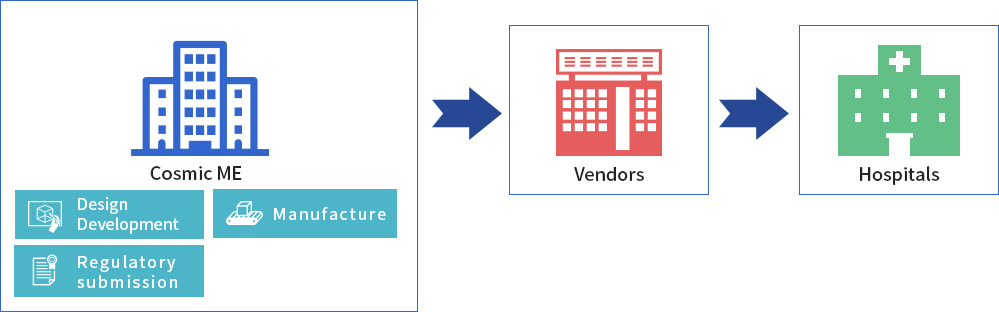
As a licensed Marketing Authorization Holder (MHA), CME can bring medical devices from concept to market. In this capacity, CME designs and develops the product, applies for regulatory approval, and also handles mass production and sales to medical device vendors.
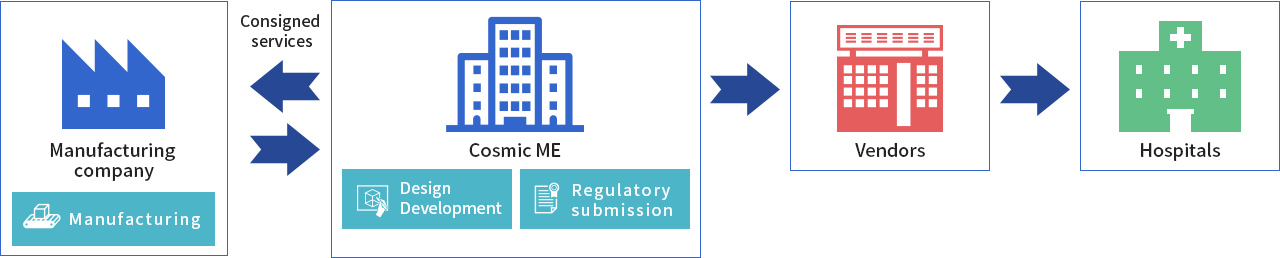
CME designs, develops and applies for regulatory approval of the medical device, in its capacity as a licensed MHA. Actual mass production is consigned to another manufacturer, but CME remains in charge of sales to medical device vendors.
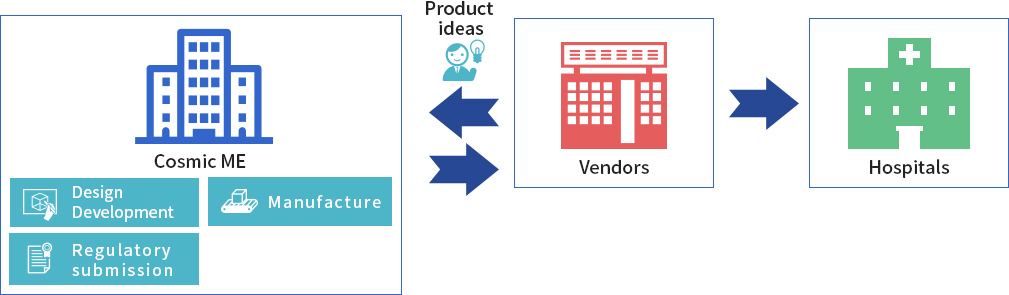
A medical device vendor or distributor engages CME to develop a product idea and manufacture the product. CME then designs and develops the medical device based on the client’s requirements. In its MHA capacity, CME applies for regulatory approval, and undertakes mass production. The finished product is sold exclusively to the medical device distributor or vender who commissioned it.

A medical device manufacturer and distributor engages CME to develop and manufacture the product. CME designs, develops and produces the medical device to client specification. The client files for regulatory approval, while CME (acting as medical device MAH) supplies the finished product on an OEM basis.
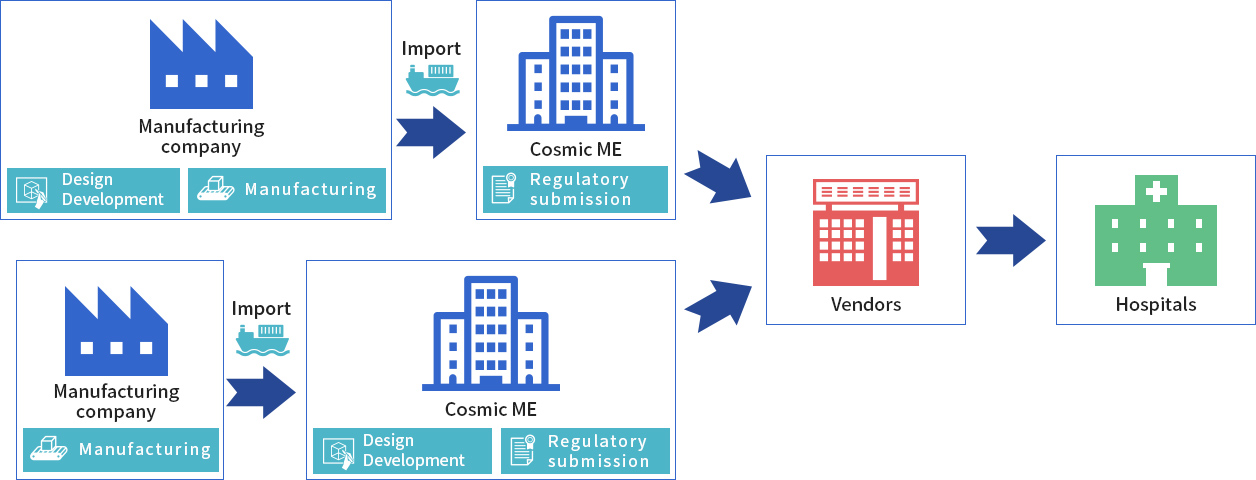
CME is designated marketing authorization holder (DMAH) by an overseas medical device manufacturer, allowing CME to import the manufacturer’s device. CME also serves as the representative service for regulatory matters in Japan.